The chapman cycle
The chapman cycle, is the continuous oxygen - ozone cycle. It is in a constant cycle with oxygen molecules and their interaction with ultraviolet rays. It is known as a cycle because it is the constant switch between 2 different molecules of oxygen. The ozone layer is created when ultraviolet rays react with oxygen molecules (O2) to create ozone (O3) and atomic oxygen (O).
The Cycle
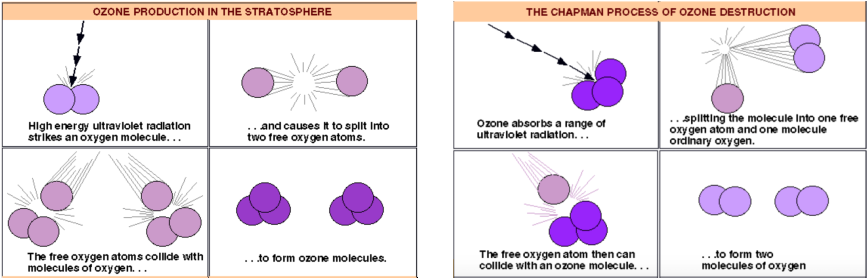
When oxygen (O2) molecules float in the atmosphere, they may be exposed to UV light which has a wavelength of 120 - 210nm. The high energy light breaks the bonds within the oxygen molecule forming two separate oxygen atoms.
O2 + UV light --> O + O
The two oxygen atoms now fly in any direction, to collide with oxygen molecules, and bond forming O3 (Ozone).
When the newly formed ozone molecule is exposed to UV radiation, it splits to form 1 free oxygen atom and an oxygen molecule.
The free oxygen atom will then collide with an ozone molecule splitting it to have an end result of 2 oxygen molecules.
O2 + UV light --> O + O
The two oxygen atoms now fly in any direction, to collide with oxygen molecules, and bond forming O3 (Ozone).
When the newly formed ozone molecule is exposed to UV radiation, it splits to form 1 free oxygen atom and an oxygen molecule.
The free oxygen atom will then collide with an ozone molecule splitting it to have an end result of 2 oxygen molecules.